Milk composition
Milk contains more water than any other element, around 87% for dairy cows. The other elements are dissolved, colloidally dispersed, and emulsified in water.
The quantities of the main milk constituents can vary considerably depending on the individual animal, its breed, stage of lactation, age and health status. Herd management practices and environmental conditions also influence milk composition. The average composition of cows milk is shown in Table 1.
Main constituent | Range (%) | Mean (%) |
Water | 85.5 – 89.5 | 87.0 |
Total solids | 10.5 – 14.5 | 13.0 |
Fat | 2.5 – 6.0 | 4.0 |
Proteins | 2.9 – 5.0 | 3.4 |
Lactose | 3.6 – 5.5 | 4.8 |
Minerals | 0.6 – 0.9 | 0.8 |
Milk fat
If milk is left to stand, a layer of cream forms on the surface. The cream differs considerably in appearance from the lower layer of skim milk. Under the microscope cream can be seen to consist of a large number of spheres of varying sizes floating in the milk. Each sphere is surrounded by a thin skin (the fat globule membrane) which acts as the emulsifying agent for the fat suspended in milk. The membrane protects the fat against enzymes and prevents the globules coalescing into butter grains. The fat is present as an oil-in-water emulsion: this emulsion can be broken by mechanical action such as shaking.
About 98% of milk fat is a mixture of triacylglycerols, with much smaller amounts of free fatty acids, mono-and diacylglycerols, phospholipids, sterols, and hydrocarbons. Milk fat also contains pigments (e.g. carotene, which gives butter its yellow colour), and waxes. Milk fat acts as a solvent for the fat-soluble vitamins A, D, E and K and also supplies essential fatty acids (linoleic, linolenic and arachidonic).
Milk proteins
Proteins perform a variety of functions in living organisms ranging from providing structure to reproduction. Milk proteins represent one of the greatest contributions of milk to human nutrition. Proteins are polymers of amino acids. Only 20 different amino acids occur, regularly in proteins. The content and sequence of amino acids in a protein therefore affect its properties. Some proteins contain substances other than amino acids, e.g. lipoproteins contain fat and protein. Such proteins are called conjugated proteins as phosphoproteins, lipoproteins and chromoproteins. The phosphate phosphate is linked chemically to phosphoproteins, the casein in milk is an example. A combination of lipid and protein forms the lipoprotein and are excellent emulsifying agents. Chromoproteins are proteins with a coloured prosthetic group and include haemoglobin and myoglobin. |
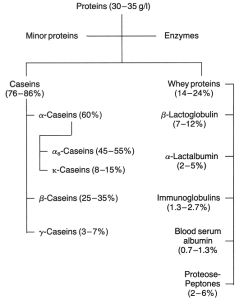
The casein
The casein is a group name for the dominant class of protein in milk. Normal bovine milk contains about 3.5% protein, ok which casein constitutes about 80%. Casein is easily separated from milk, either by acid precipitation or by adding rennin. In cheesemaking most of the casein is recovered with the milk fat. Casein can also be recovered from skim milk as a separate product.
Casein is dispersed in milk in the form of micelles. The micelles are stabilised by the Κ-casein. Caseins are hydrophobic but Κ-casein contains a hydrophilic portion known as the glycomacropeptide and it is this that stabilises the micelles. The structure of the micelles is not fully understood.
When the pH of milk is changed, the acidic or basic groups of the proteins will be neutralised. At the pH at which the positive charge on a protein equals exactly the negative charge, the net total charge of the protein is zero. This pH is called the isoelectric point of the protein (pH 4.6 for casein). If an acid is added to milk, or if acid-producing bacteria are allowed to grow in milk, the pH falls. As the pH falls the charge on casein falls and it precipitates. Hence milk curdles as it sours, or the casein precipitates more completely at low pH.
The whey proteins
The whey proteins are also made up of a number of distinct proteins as shown in the scheme in Figure 1. Whey protein comprises the group of proteins in whey during the cheesemaking process. Whey protein also contains fragments of casein molecules.
After the fat and casein have been removed from milk, one is left with whey, which contains the soluble milk salts, milk sugar and the remainder of the milk proteins. Like the proteins in eggs, whey proteins can be coagulated by heat. When coagulated, they can be recovered with caseins in the manufacture of acid-type cheeses. The whey proteins are made up of a number of distinct proteins, the most important of which are b-lactoglobulin and lactoglobulin. b-lactoglobulin accounts for about 50% of the whey proteins, and has a high content of essential amino acids. It forms a complex with Κ-casein when milk is heated to more than 75°C, and this complex affects the functional properties of milk. Denaturation of b-lactoglobulin causes the cooked flavour of heated milk.
Other milk proteins
In addition to the major protein fractions outlined, milk contains a number of enzymes. The main enzymes present are lipases, which cause rancidity, particularly in homogenized milk, and phosphatase enzymes, which catalyze the hydrolysis of organic phosphates. Measuring the inactivation of alkaline phosphatase is a method of testing the effectiveness of pasteurization of milk.
Peroxidase enzymes, which catalyze the breakdown of hydrogen peroxide to water and oxygen, are also present. Lactoperoxidase can be activated and use is made of this for milk preservation.
Milk also contains protease enzymes, which catalyze the hydrolysis of proteins, and lactalbumin, bovine serum albumin, the immune globulins and lactoferrin, which protect the young calf against infection.
Milk carbohydrates
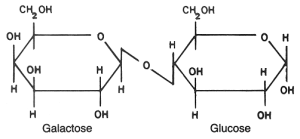
Lactose is the major carbohydrate fraction in milk. It is made up of two sugars, glucose and galactose (Figure 2). The average lactose content of milk varies between 4.7 and 4.9%, though milk from individual cows may vary more. Mastitis reduces lactose secretion. |
Lactose is a source of energy for the young calf, and provides 4 calories/g of lactose metabolized. It is less soluble in water than sucrose and is also less sweet. It can be broken down to glucose and galactose by bacteria that have the enzyme b-galactosidase. The glucose and galactose can then be fermented to lactic acid. This occurs when milk goes sour. Under controlled conditions they can also be fermented to other acids to give a desired flavour, such as propionic acid fermentation in Swiss-cheese manufacture.
Lactose is present in milk in molecular solution. In cheesemaking lactose remains in the whey fraction. It has been recovered from whey for use in the pharmaceutical industry, where its low solubility in water makes it suitable for coating tablets. It is used to fortify baby-food formula. Lactose can be sprayed on silage to increase the rate of acid development in silage fermentation. It can be converted into ethanol using certain strains of yeast, and the yeast biomass recovered and used as animal feed. However, these processes are expensive and a large throughput is necessary for them to be profitable. For smallholders, whey is best used as a food without any further processing.
Heating milk to above 100oC causes lactose to combine irreversibly with the milk proteins. This reduces the nutritional value of the milk and also turns it brown.
Because lactose is not as soluble in water as sucrose, adding sucrose to milk forces lactose out of solution and it crystallizes. This causes sandiness in such products as ice cream. Special processing is required to crystallize lactose when manufacturing products such as instant skim milk powders.
Some people are unable to metabolize lactose and suffer from an allergy as a result. Pre-treatment of milk with lactase enzyme breaks down the lactose and helps overcome this difficulty.
In addition to lactose, milk contains traces of glucose and galactose. Carbohydrates are also present in association with protein. Κ-casein, which stabilizes the casein system, is a carbohydrate-containing protein.
Milk salts
Milk salts are mainly chlorides, phosphates and citrates of sodium, calcium and magnesium. Although salts comprise less than 1 % of the milk they influence its rate of coagulation and other functional properties. Some salts are present in true solution. The physical state of other salts is not fully understood. Calcium, magnesium, phosphorous and citrate are distributed between the soluble and colloidal phases (Table 2). Their equilibria are altered by heating, cooling and by a change in pH.
In addition to the major salts, milk also contains trace elements. Some elements come to the milk from feeds, but milking utensils and equipment are important sources of such elements as copper, iron, nickel and zinc.
Total | Dissolved | Colloidal | |
(mg/100 ml of milk) | |||
Calcium | 1320.1 | 51.8 | 80.3 |
Magnesium | 10.8 | 7.9 | 2.9 |
Total phosphorus | 95.8 | 36.3 | 59.6 |
Citrate | 156.6 | 141.6 | 15.0 |
Milk vitamins
Milk contains the fat-soluble vitamins A, D, E and K in association with the fat fraction and water-soluble vitamins B complex and C in association with the water phase. Vitamins are unstable and processing can therefore reduce the effective vitamin content of milk.